What Is Temperature?
Temperature is an indicator that shows the state of a substance such as hot and cold. Temperature is expressed in units such as Kelvin (K), Celsius (°C), and Fahrenheit (°F).
This section explains the mechanism where heat, which is closely related to temperature, is generated and the units of measurement.
Difference between temperature and heat
First, let’s figure out what temperature and heat are.
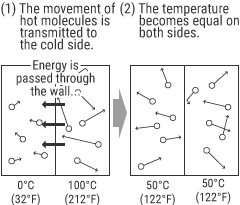
All substances (molecules) have energy generated by vibration or other factors. “Heat” is a form of energy.
Then, what is the difference between “temperature” and “heat”?
“Temperature” is a scale to measure “heat” within a substance—namely, a quantification of the state of the heat energy.
Because heat energy moves between substances having different temperatures, a hot substance and a cold substance come to the same temperature after being kept in contact with each other.
Units of temperature
There are various units of temperature, including degrees Celsius (°C), degrees Fahrenheit (°F), and Kelvin (K, absolute temperature, which is mainly used in thermodynamics). These three units of temperature are compared as follows.
| Kelvin | Degrees Celsius | Degrees Fahrenheit | |
|---|---|---|---|
|
Absolute zero
|
0K
|
-273.15°C
|
-459.67°F
|
|
Fahrenheit’s ice/salt mixture
|
255.37K
|
-17.78°C
|
0°F
|
|
Ice melts (in standard state)
|
273.15K
|
0°C
|
32°F
|
|
Water boils (in standard state)
|
373.15K
|
100°C
|
212°F
|





